Early visual symptoms aren’t enough. Thyretain is the only FDA-cleared bioassay that specifically detects the TSI antibodies directly linked with Graves’ disease.
Diagnosing Graves’ disease
2016 New ATA Hyperthyroid Guidelines
*RECOMMENDATION 1
The etiology of thyrotoxicosis should be determined. If the diagnosis is not apparent based on the clinical presentation and initial biochemical evaluation, diagnostic testing is indicated and can include, depending on available expertise and resources, (1) measurement of TRAb, (2) determination of the radioactive iodine uptake (RAIU), or (3) measurement of thyroidal blood flow on ultrasonography. An iodine-123 or technetium-99m pertechnetate scan should be obtained when the clinical presentation suggests a toxic adenoma or toxic multinodular goiter. Strong recommendation, moderate-quality evidence.
The Recommendation to test for the thyroid receptor antibodies is here mentioned for the first time in the guidelines.
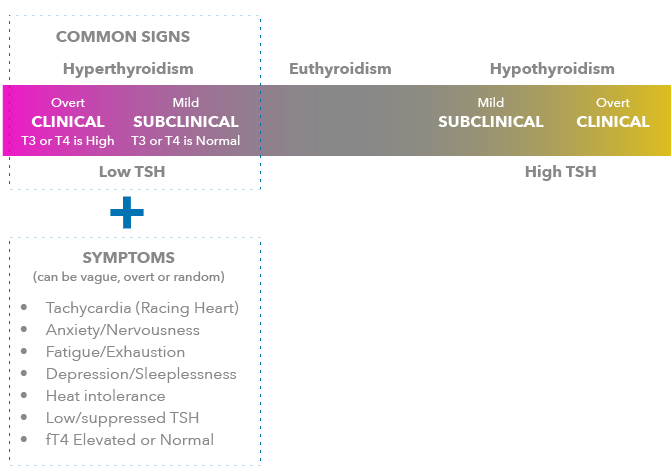
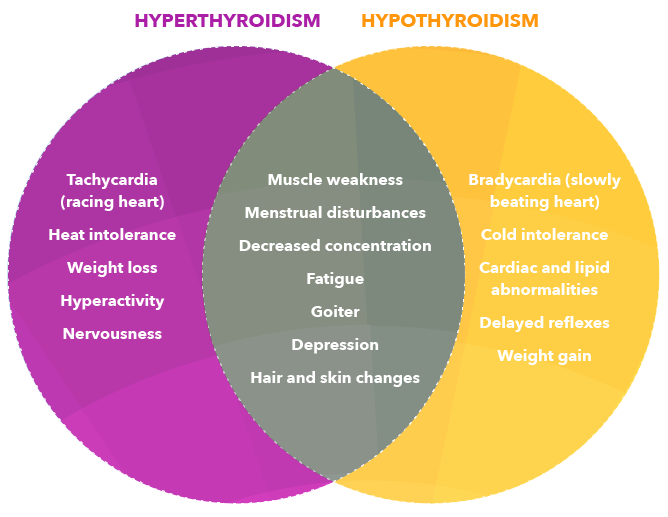
- Hyperthyroidism is typically characterized by signs like low or suppressed thyroid stimulating hormone (TSH) levels and elevated T4 and/or T3 values.
- Over 70% of these hyperthyroid patients have the autoimmune disease called Graves’ disease.
- The causative agent of Graves’ disease is thyroid stimulating immunoglobulin (TSI).
- Elevated levels of TSI in a patient’s bloodstream can be detected using the Thyretain TSI Reporter BioAssay. Positive results can provide healthcare providers with an important specific aid in the diagnosis of Graves’ disease.
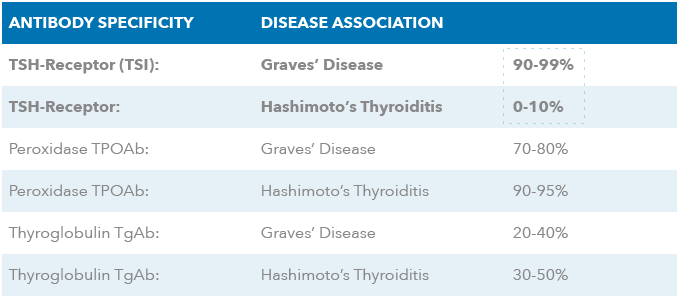
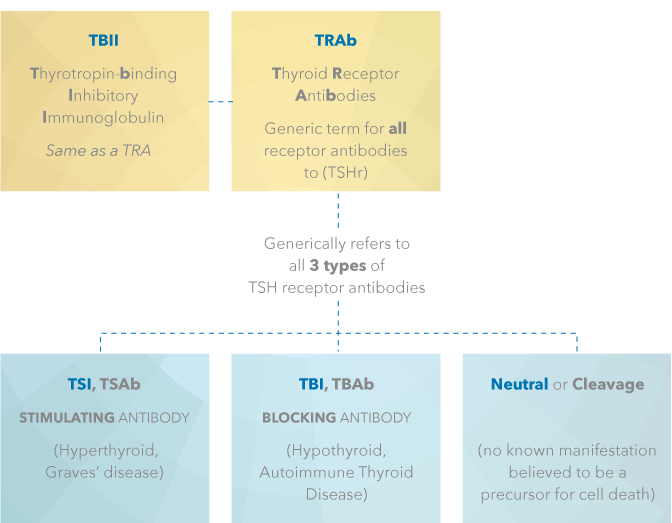
Generic and Specific Descriptions of Thyroid Stimulating Hormone Receptor (TSHr) Antibodies
just the target antibodies
The Most Common Autoimmune Disorder in the United States
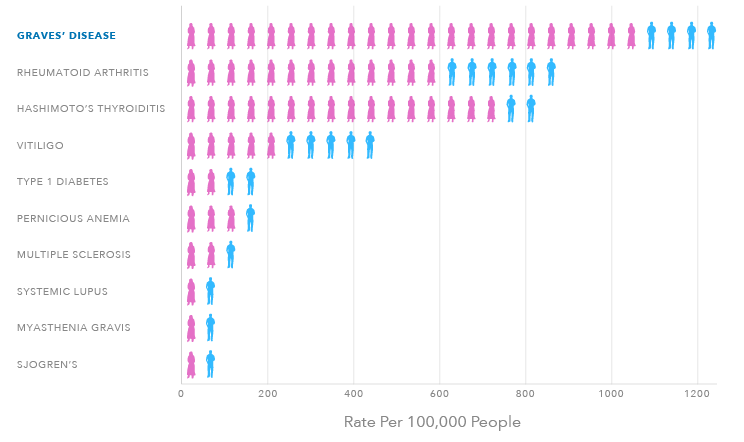
3 MILLION
PEOPLE IN THE U.S.
12,000
CASES PER MONTH
8:1
WOMEN TO MEN
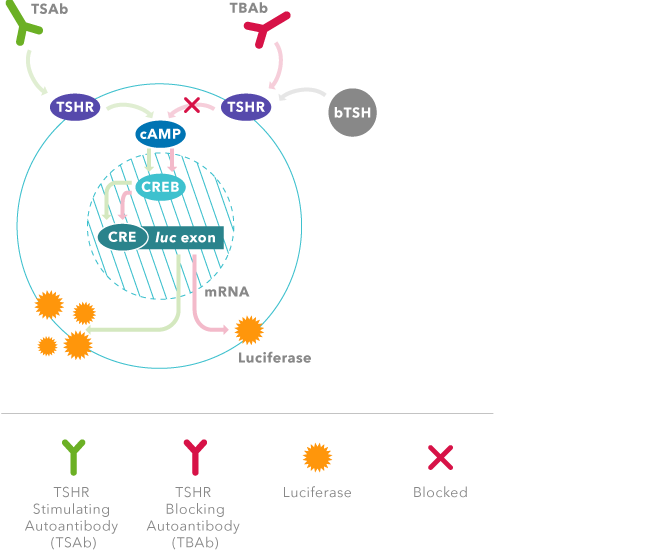
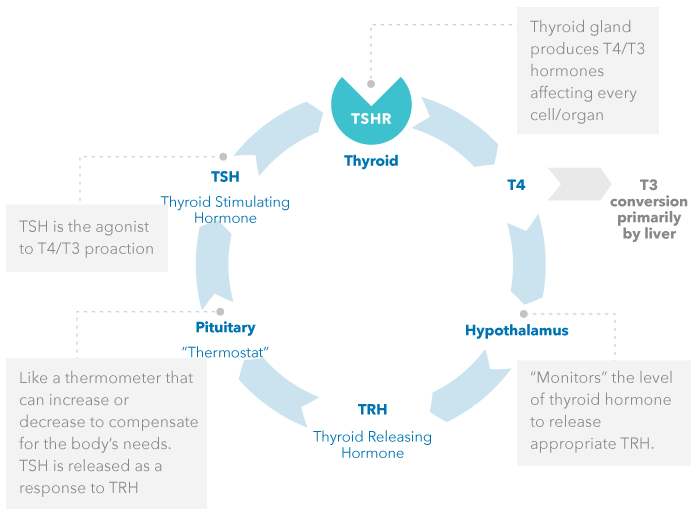
TSI Function
In normal thyroid metabolism, thyroid hormone production is tightly regulated by the hypothalamic-pituitary-thyroid axis.
NORMAL THYROID FUNCTION
Negative feedback loop
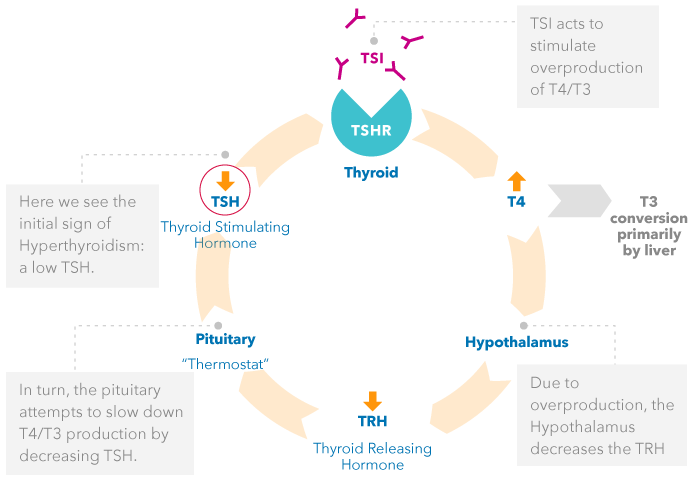
Graves’ disease is an autoimmune disorder that primarily affects the thyroid. The Thyroid is responding to the stimulation of TSI and is not “malfunctioning” per se. Thyroid Stimulating Immunoglobulin (TSI) mimics the action of TSH on the TSH receptor, in that it causes T4 to be over produced. TSI is not regulated by the same negative feedback system.
EFFECTS OF TSI – PATIENTS WITH GRAVES’ DISEASE
How can the Thyretain BioAssay Help?
Find the specific culprit behind the disease.
Based on a novel genetically engineered cell line, Thyretain TSI BioAssay detects only the TSI, or stimulating antibodies, that cause the disease. The engineered cell technology also enhances the sensitivity for detecting TSI , providing improved results over currently available non-specific assays.
Graves’ Disease is an Autoimmune Disorder
Thyretain Can Quickly Help Differentiate Graves’ Disease Induced Autoimmune Hyperthroidism from Non-Autoimmune Hyperthroidism
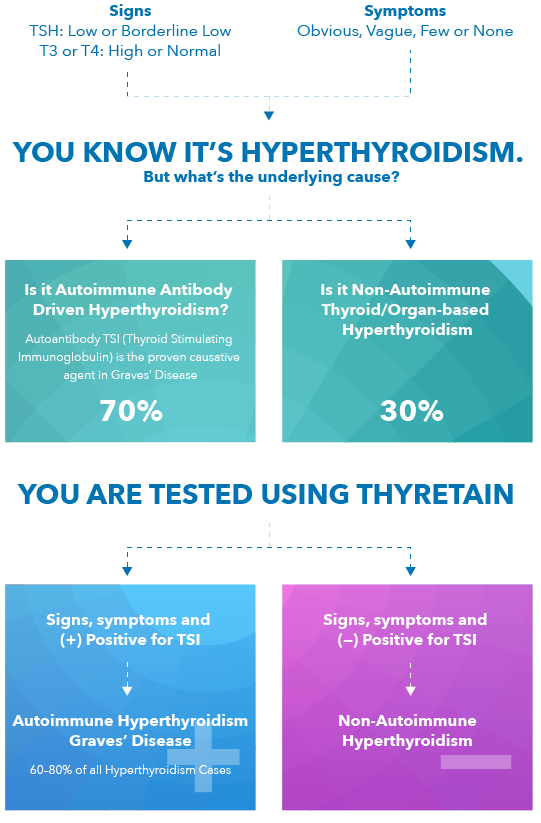
- TSI is the autoantibody that stimulates TSH receptors – and is the cause of Graves’ Disease. 1
- Quidel’s TSI Reporter Assay (Thyretain) is the only FDA-cleared bioassay that specifically detects TSI.
- If a positive TSI result, patient has autoimmune disease; a differential diagnosis of Graves’ can be confirmed. 1,2
Where do I order Thyretain TSI BioAssay?
The Thyretain TSI BioAssay is available through the following laboratories
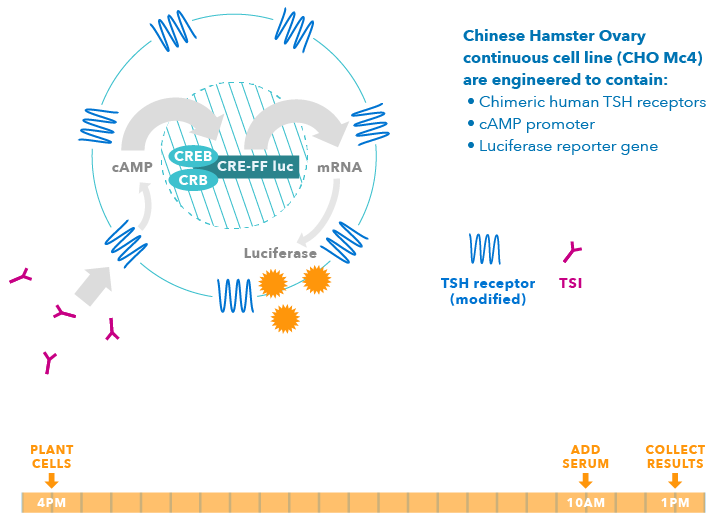
Detecting TSI Using Thyretain
Thyretain is a living bioassay system. It has been genetically engineered with chimeric human TSH receptors that are linked to a luciferase gene (firefly light) and will produce light indicating the presence or absence of TSI antibodies.
As a living system, these cells respond to TSI and have been shown to correlate with disease severity and extrathyroidal manifestations (i.e., eye or skin disease associated with TSI).
Only One Day Turnaround Time in the Lab
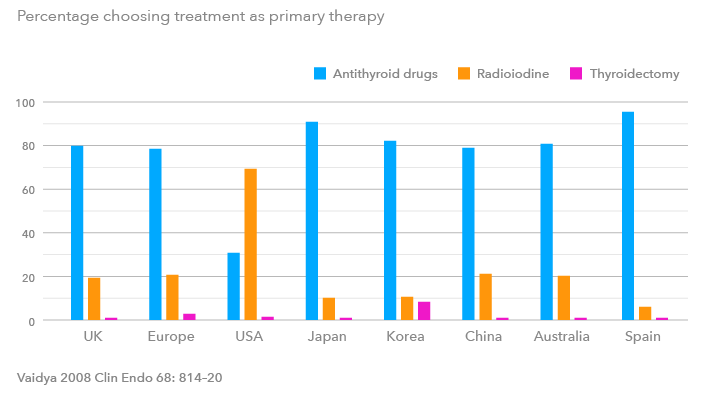
Treatment options
(Treatment is a personal choice and Quidel does not advocate
one therapy over another)
Medical Management Therapy
Antithyroid drugs (ATD) are suppressive drugs meant to inhibit the production of thyroid hormones T4 and/or T3 in an effort to treat the symptoms of Graves’ disease. These medications include Methimazole (MMI), and propylthiouracil (PTU). MMI and PTU act by inhibiting the synthesis (the production) of thyroid hormone. PTU use has been stopped especially in children, due to effects on liver function. MMI is widely used and does not carry this restriction. Medical management has a success rate of ~45-50% using current medical management guidelines. Patients retain their thyroid and may have remission of the autoimmune response. Those patients who do not respond to the ATD therapy can either continue the ATD or chose the definitive cure options listed below.
There are some side effects when using ATDs that can occur. Most are mild (rash occurs in 3-4% of patients), but one in particular is sever (agranulocytosis occurs in ~0.3% of patients). This is extremely rare and corrected by stopping the ATD therapy.
DEFINITIVE THERAPIES
Radioiodine Ablation Therapy
Iodine-131 (Radioiodine) is given orally to destroy the function of a hyperactive gland. Usually one dose is sufficient but some patients might require more than one treatment. The radioactive iodine is picked up by the active cells in the thyroid, which use iodine to produce T4, and destroys these cells.
Radioiodine is an ablative approach and is considered the definitive cure for hyperthyroidism brought about by eliminating most if not all of the target organ; however, >80% of patients develop permanent hypothyroidism soon after treatment and require lifelong synthetic hormone replacement. Hypothyroidism is believed to be an easier disease to manage than hyperthyroidism.
Theoretically the radioiodine could be dosed to only destroy part of the thyroid gland, but the dosage is extremely difficult to determine, and if thyroid tissue remains, the body can still produce TSI against any remnant tissue. These antibodies can still cause Graves’ eye disease even after ablative therapy. For incompletely ablated women in their child bearing years these antibodies could be passed across the placenta during pregnancy, leading to the child developing Graves’ disease. This has been addressed in the ATA and AACE guidelines for treating pregnant women and those considering pregnancy who have undergone this treatment. (see Graves’ disease and Pregnancy)
Surgery
This option is also considered a definitive cure for Graves’ disease. For patients who cannot tolerate medicines or who are allergic to or decline iodine-131, a surgical treatment option is available. Full or partial thyroidectomy is used uncommonly in the US and is not considered a primary option for Graves’ disease treatment, due to risks from surgery and anesthesia. If this option is chosen, it is important to select a surgeon with sufficient skill in thyroid surgery specifically.
TREATMENT CAN VARY BY REGION
TSI antibodies can pass through the placenta and affect the fetus. As a result, the ATA/AACE issued the following guidance:
- Recommendation 74: TSH receptor (TSH-R) antibody levels should be measured to differentiate the etiology of hyperthyroidism in pregnancy
- Recommendation 75: Patients who were treated for Graves’ disease prior to pregnancy should have TSH-R antibody levels measured initially during the first trimester and, if elevated, again at 22-26 weeks of gestation
- Recommendation 76: Patients diagnosed with Graves’ disease during pregnancy should have TSH-R antibody levels measured at diagnosis and again at 22-26 weeks of gestation
- Recommendation 77: TSH-R antibody levels measured at 22-26 wks of gestation should be used to guide decisions regarding neonatal monitoring
The American Thyroid Association recommends that the following high-risk women be screened for thyroid disease either prior to becoming pregnant, or as soon as feasible once a woman becomes pregnant:
- Women with a history of thyroid disease or thyroid surgery.
- Women with a family history of thyroid disease
- Women with a goiter
- Women with known thyroid antibodies
- Women with symptoms or clinical signs of hyperthyroidism or hypothyroidism
- Women with Type I diabetes mellitus
- Women with other autoimmune disorders
- Women with infertility
- Women with previous therapeutic head or neck irradiation
- Women with a history of miscarriage or preterm delivery